Hi everyone,
I had my 31st Tysabri infusion last Wednesday. My regular infusion nurse was not there that day, nor was her backup, so a lesser experienced infusion nurse had to stick me three times to get a good line in (owie!)
Below are some Tysabri charts/graphs that an acquaintance of mine compiled from Tysabri patient reports being posted on a MS forum that I belong to. A friend of mine sent them to me and I am on posting them on my blog as well.
Also, below is my reply to a post from a patient that is, how should I say this politely, not very Tysabri friendly and never has been... she is always posting negative and erroneous misconceptions about Tysabri on various MS boards.
Anyway, hopefully each of you will have a good week.
((((hugs))))
Love, Lauren :)
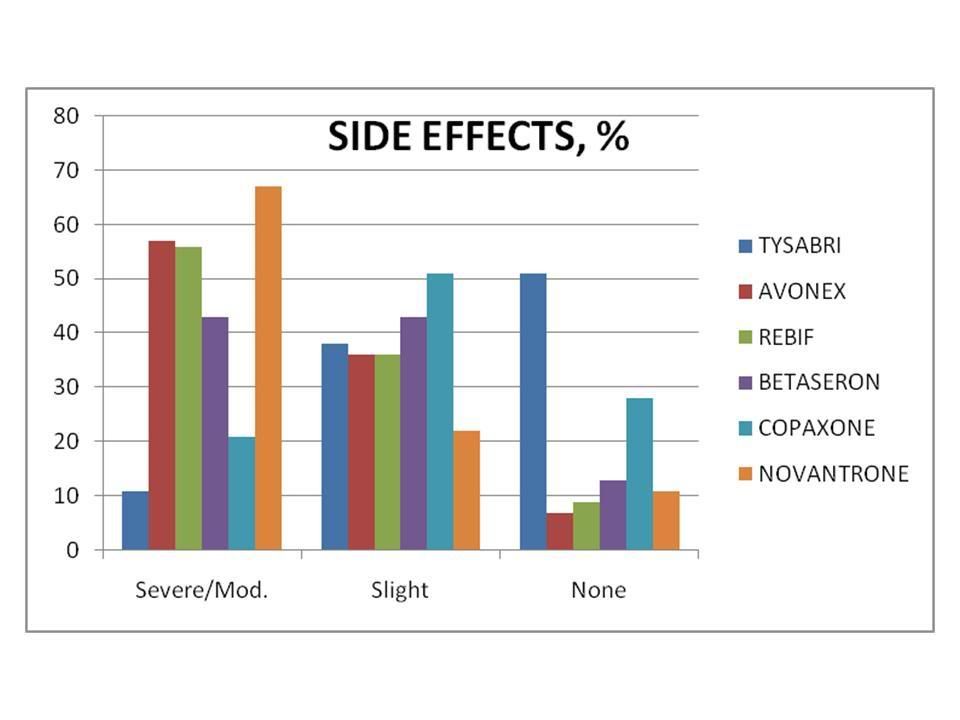
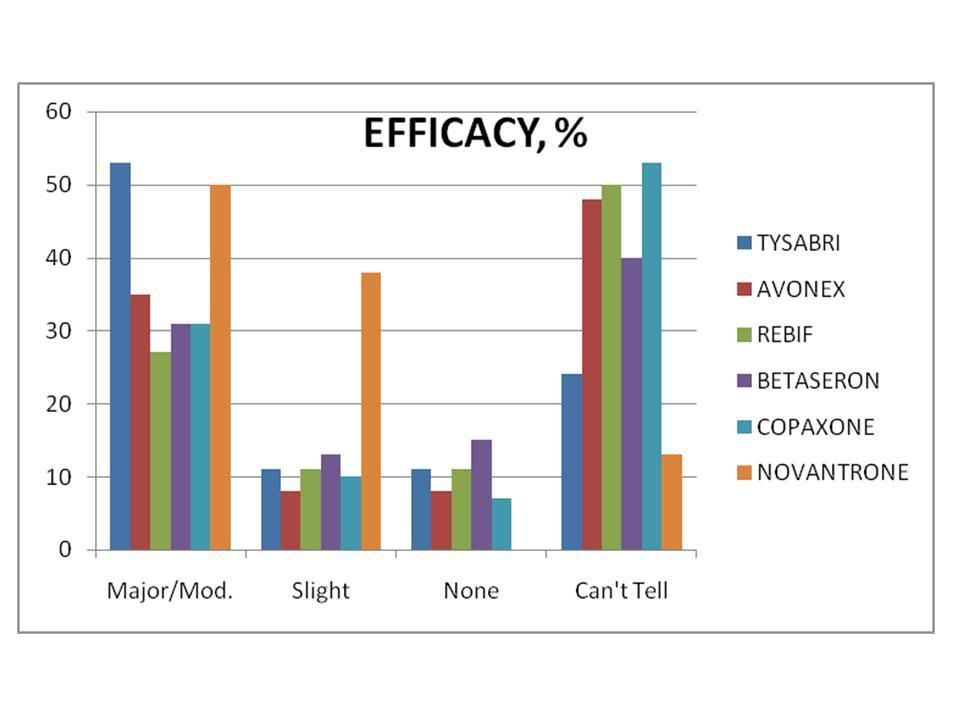
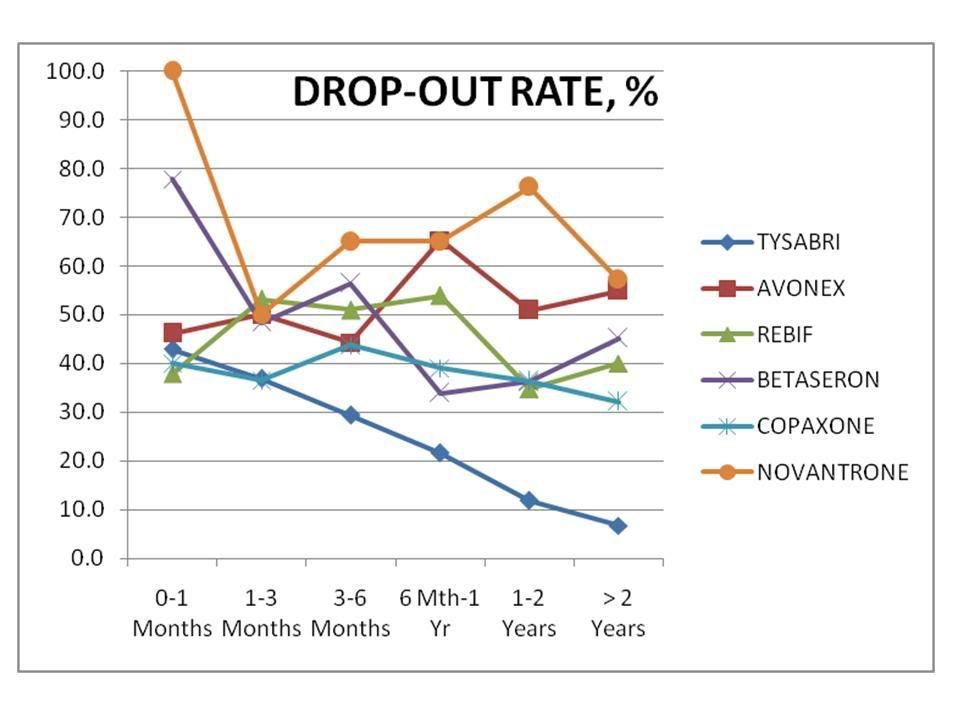
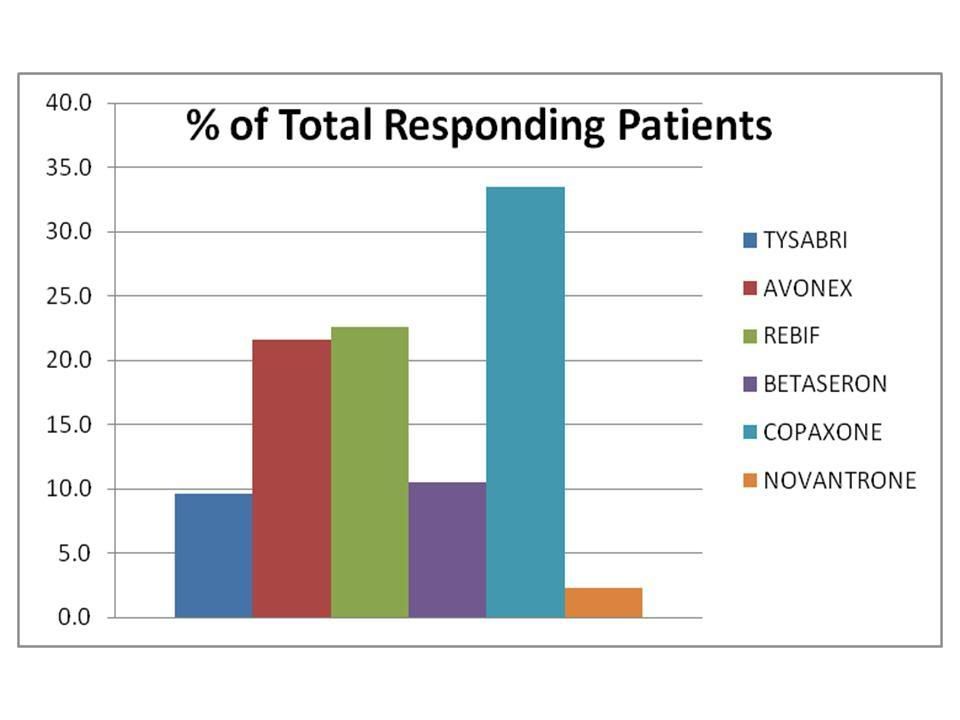
(Percentage of patients responding to various "Tysabri Polls”)
This reply is in response to the post by XXX Re: the IRIS condition and other various misconceptions/assumptions...,
The patient who died was a United States patient who had received 14 monthly infusions of Tysabri as a stand-alone treatment. Previously she had received other therapies, including methotrexate for her RA condition... methotrexate has been specifically linked to causing PML.
Unfortunately, methotrexate has been positively associated with PML because of its extremely strong immunosuppressive actions. See page 1 of the following NEJM article, which states the following:
"Progressive multifocal leukoencephalopathy (pml) is a rare, oligodendroglial infection caused by the polyomavirus JC virus. It [PML] usually occurs in people infected with the human immunodeficiency virus (HIV), but it has also been reported in immunocompromised patients receiving prolonged treatment with methotrexate, cyclophosphamide [a.k.a. Cytoxan], and azathioprine [a.k.a. Imuran].." Here is the link for the New England Journal of Medicine article:
http://content.nejm.org/cgi/reprint/353/4/375.pdf (NEJM)
After developing the brain infection, the patient was treated with a procedure known as plasmapheresis, in which blood is removed, cleared of the drug, and replaced.While the U.S. patient died, two patients who developed PML in Europe, and whose cases were announced in July, appear to be recovering following treatment, even though one had not been expected to survive. Earlier this month, Biogen announced that a fourth patient had developed PML. This patient, in Europe, is still alive.The European patient that had not been expected to survive had developed a condition known as immune reconstitution inflammatory syndrome, or IRIS. This occurs when the immune system, in eliminating an infection, produces an excessive inflammatory response that can worsen symptoms.
From an e-mail reply that I received from the Senior Director of Research Information of the NMSS which posted information/their opinions regarding IRIS, she stated "What is not known is whether IRIS would have occurred whether PLEX was performed or not. Further experience will probably answer that and many other questions as time goes by....Biogen Idec is to be applauded for supporting research into PML and possible ways to quickly clear the system of their drug when it's deemed necessary."
"Geoff Meacham, an analyst at J.P. Morgan, said it seems likely the U.S. patient, having gone through plasmapheresis, died of IRIS... ", Mr. Meacham is a financial analyst, not a doctor/researcher.
Drugs that have been known to cause PML include Imuran/Azathioprine, regular steroid use, Rituxin, Remicade, CellCept, methotrexate, Raptiva, etc, and to unfairly single out Tysabri causing PML/IRIS is ludicrous.
All of these patients that developed PMLwere previously treated with one of the following: Remicade, Imuran/Azathioprine, methotrexate, regular steroid use, and possibly CellCept in the latest PML case. So as you can see, all of these patients received strong immune suppressing medications which can last in the body for years (even though previously discontinued by the patient) prior to starting Tysabri therapy.
As of end of December 2008 there were approximately 37,600 patients currently on therapy. A total of 52,900 have been treated with Tysabri at some point in time (clinical trials, first launch, second launch), so the 1:1000 risk of developing PML is still well within the guidelines as listed on the label.
And Jan, you're more than welcome for the information 
Lauren


